Covalent Bond – CrackChemistry
First of all, You must be familiar with what is bond ?
The bond is usually refers to be electrostatic attraction between two ions or elements via two electrons is called as Bond.
Covalent bond :-
Did you think that elements love sharing ?
Well,yes
They always love to share their electrons to get their Octet State
If you write Electronic Configuration of Oxygen (⁸O)
1s² 2s² 2p⁴
To complete its Octet it has to find 2 more electrons anyhow. And
If you write Electronic Configuration of Hydrogen (¹H)
1s¹
It has single electron. For Hydrogen to achieve its octet is next to impossible because it orbit allow only 2 electrons.
So there is a Relaxation in Octet for Hydrogen, that is it has completely fill its outer most orbit.
 |
| Bonding in water Molecule |
While looking at this complicated Situation of Oxygen & Hydrogen , Both has Lesser amount of electrons. Both are in need of electrons. So they’re Decided to Share electrons and Help each other to achieve Octet.
 |
| Types of covalent bond |
The Shared pair of electrons in bond is called as Bonding Electrons.
• When there was a single shared pair of electrons present between 2 atoms it forms Single bond.
• When there is two shared pair of electrons present between 2 atoms, there will be Double Bond
• When there is three shared pair of electrons there will be Triple bond
The mutual sharing of electrons of elements to form bond is known as Covalent Bond.
Note :-
As you see above, Oxygen and hydrogen shared single pair (2 times) so it forms Single bonds.
So now, the Oxygen and Hydrogen are not individual Elements. They combined together and They formed as H₂O molecule.
Both Oxygen and Hydrogen are present in Gaseous state. But as combine together they form the most abundant substance and very important substance of survival in earth and our body.
Yes, you guessed right…
H₂O is Water.
Whenever element combine together and formed as the “Molecule“. Molecule has its own property and characteristics are totally different form the individual elements. As you see Water is liquid but both Oxygen and Hydrogen present as Gases.
How to write that how many elements bond together and how many pair of electrons they share ?
There are few scientific ways to find out in precise way.
Method :-
It shows the number of elements and their Ratio.
As formula of Water (H₂O) tells us that a molecule of water is formed by two atoms of Hydrogen with one atom of Oxygen.
Lewis Dot Structure :-
It was named after scientist Gilbert Newton Lewis.In this method,Electrons of outermost orbits are shown as dot in symbol of the element
 |
| Lewis dot structure of Water Molecule |
Kekule Structure :-
In this method, Bonding Electrons of the element are shown in Lines.
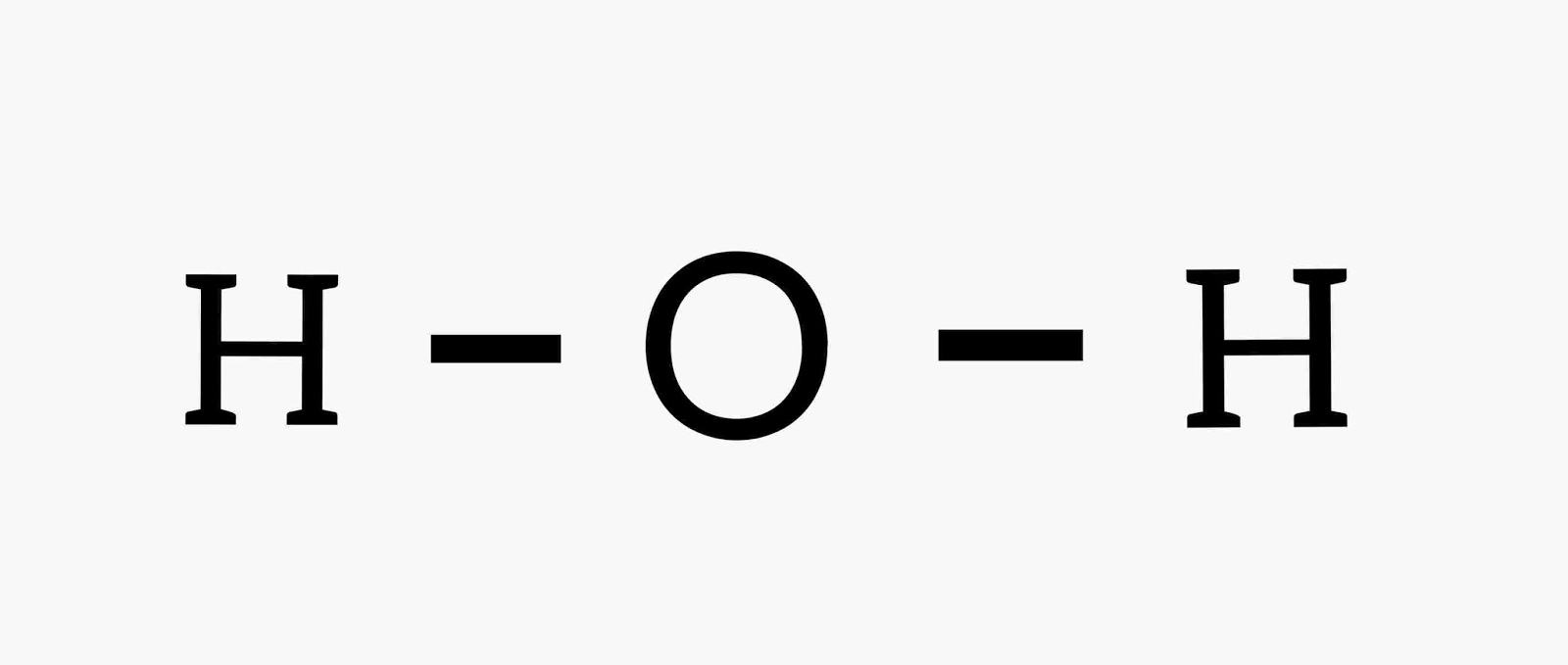 |
| Kekule structure |
End —
Crack Chemistry – Do follow
Originally posted 2022-10-28 09:24:00.
