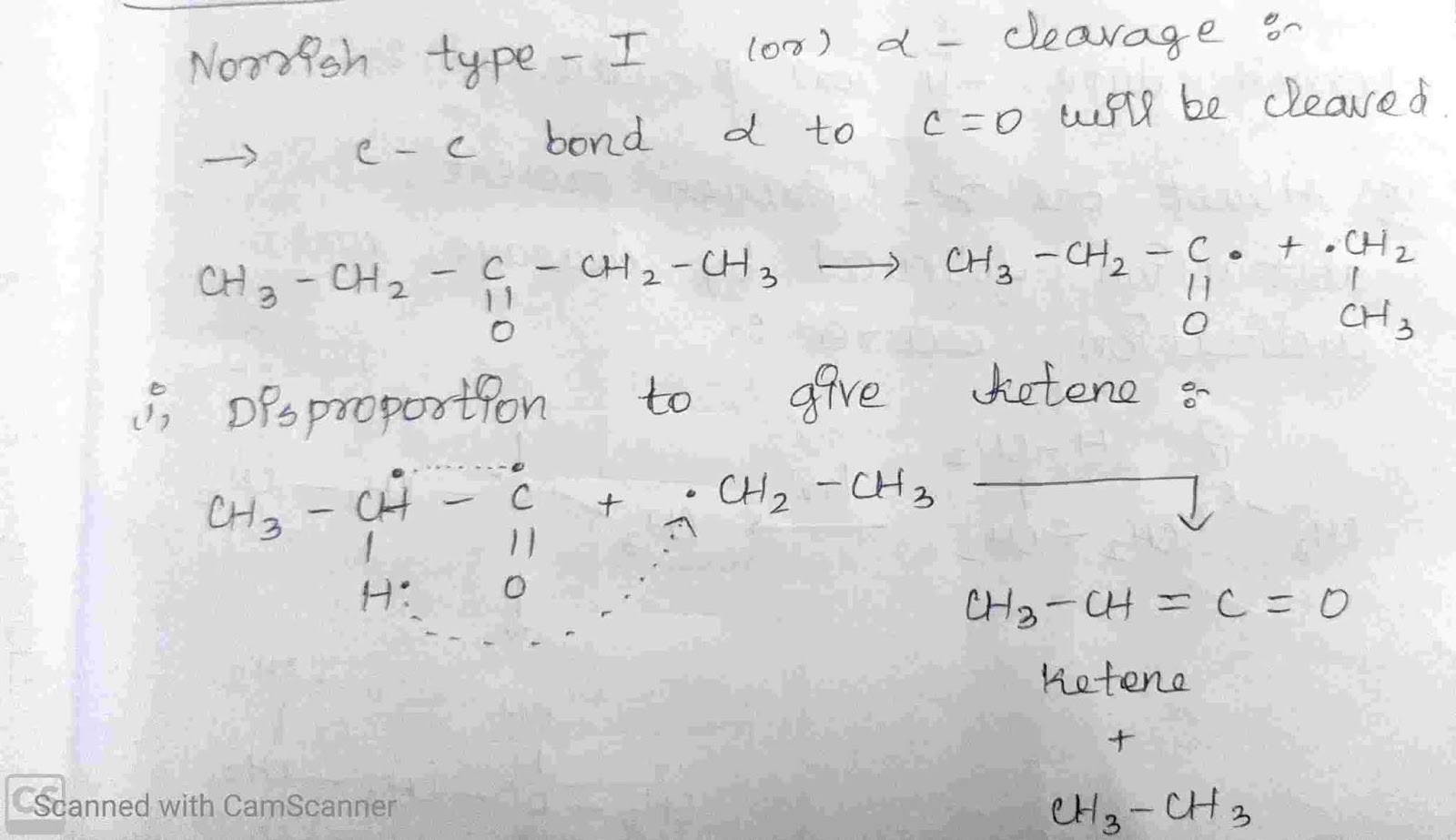Photochemistry Notes
Here I have attached Photochemistry Notes in this article, make use of that – Jablonsky diagram – Important Reactions in Photochemistry
In this blog, we’re going to see about the basics of photochemistry,
1. What is Photochemistry ?
2. What is Electronic excitation ?
3.Jablonsky diagram
4.Norrish Type – I (or) Alpha Cleavage
5.Norrish Type – II (or) Beta Cleavage
before we start, I hope everyone knows about molecular orbital theory..
Lets start…,
Photochemistry :-
(i) The study of reactions brought about by the action of Light.
(ii) The reactions that occurred by absorption (or) emission of light in chemistry is generally termed as Photochemistry.
(iii) When a Molecule absorbs Radiation, it Gets Excited.it cant be stable in Ground state and it also most prefer to excited to higher energy level. (i.e) Anti bonding orbitals – MOLECULAR ORBITAL THEORY
Photochemistry notes :
Electronic Excitation :-
There are Four types of Excitation, they are
(i). Sigma to Sigma*(star) – Antibonding
Example :- Alkanes
(ii).Non-bonding to Sigma*(star) -Antibonding. This mostly happens in hetero atoms ( Atoms/Molecules having lone pair of electrons)
Example :- oxygen & nitrogen atoms of alcohols, amines etc.,
(iii). Pi to Pi* (star) – Antibonding. This excitation happens in the Compounds having Pi bond or Pi electrons
Example :- Alkenes, ketones, esters etc.,
(iv) Non bonding to Pi* (star) – Antibonding
Example :- Aldehydes, ketones etc.,
Jablonsky Diagram :-
This diagram is very important diagram of photochemistry. The core photochemistry is depend upon this jablonsky diagram
• It simply explains about the activities of the particular excited electron in the excited state level.
• It also explains about how the electron stay in the anti-bonding orbital and how it is return to ground state and more
The primary criteria for this diagram is the atom/Molecule must absorb the light energy and exciting to the higher energy level.
1.Non – Radioactive Transition :-
Return of the activities molecule from higher excitation state to first excitation state. This phenomenon is known as Integral Conversation ( IC )
It also posses Intersystem Crossing (ISC)
transition between Different Spin of electron
Time in Seconds :- 10^-11 sec.
Occurring of IC :- S3 to S1 , T2 to T1
Occurring of ISC :- S2 to T2
2. Radioactive Transition :-
The transition taking place in S1 to S0 and T1 to S0 along with emission of radiation ( Radioactive)
• S1 to S0 in 10^-8 Sec and it posses Fluorescence
• T1 to S0 in 10^-3 and posses Phosphorescence
• Chemical reaction can also occur.
 |
| States and Processes of Jablonsky Diagram |
Important Reactions in Photochemistry :-
Norrish Type – I (or) Alpha Cleavage :-
Norrish Type – II (or) Beta Cleavage :-
I think these information’s are enough to know about Photochemistry, for more understanding of photochemistry you should read about what is Singlet, triplet states, stark-einstein equation, quantum yields etc.,
Those topic will help you a lot to understand the Photochemistry
For any queries, Feel free to comment
Thank you
Crack Chemistry
Crack Chemistry – Do follow
Originally posted 2022-10-28 04:49:00.